Ph.D. Program in Oceanography and Global Change at the Canary Islands, Spain
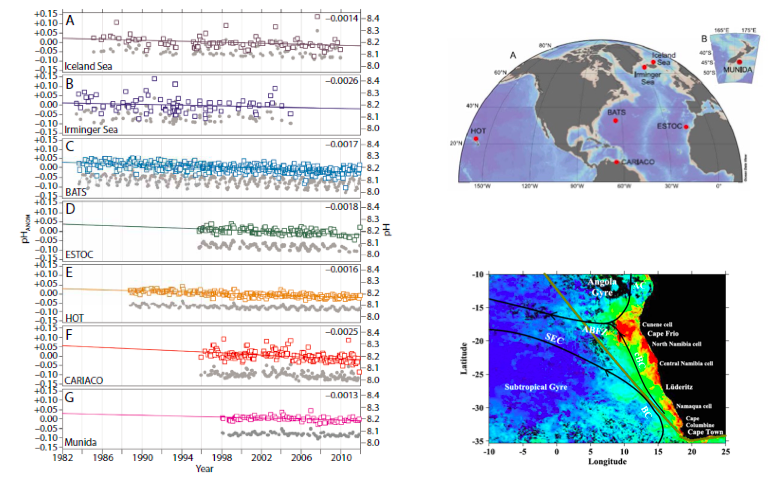
The uptake of anthropogenic CO2 from the atmosphere by the global ocean has significant implications for surface ocean chemistry, individual marine organisms and ocean ecosystems. Calculations based on measurements of the ocean surface and coupled with existing knowledge of the ocean chemistry, indicate that the uptake of CO2 after pre-industrial time has led to a reduction of the surface seawater pH of 0.1 units over its pre-industrial values. This is equivalent to a 30% increase in the concentration of hydrogen ions. By the end of the century, the models predict that pH will decrease another 0.3-0.4 units, the dissolved inorganic carbon, CT, may increase by over 12 %, and the carbonate ion concentration may decrease by 60%.
In order to study how the pH and carbonate system of the oceans are responding to the CO2 increase due to anthropogenic activities, two different approaches are possible and are applied by QUIMA group
- First, sustained time-series observations and VOS surface pCO2 measurements that provide data, not only about ocean physics and biogeochemistry but also information about processes such as ocean acidification and deoxygenation. The longest ocean CO2 time-series sites (about fifteen to thirty years) and sustained VOS lines can be considered in order to establish seasonal and some interannual changes in seawater CO2-carbonate chemistry that reflect changes in the natural carbon cycle and anthropogenic perturbation.
- Second, lower frequency sampling along ocean sections annually reoccupied and/or episodic reoccupied through projects like WOCE and CLIVAR/CO2 repeat hydrography where the carbonate system variables, pH total alkalinity and total dissolved inorganic carbon in the water column, are determined with high accuracy methods in order to provide the nowadays and future contents of natural and anthropogenic carbon budget and storage.